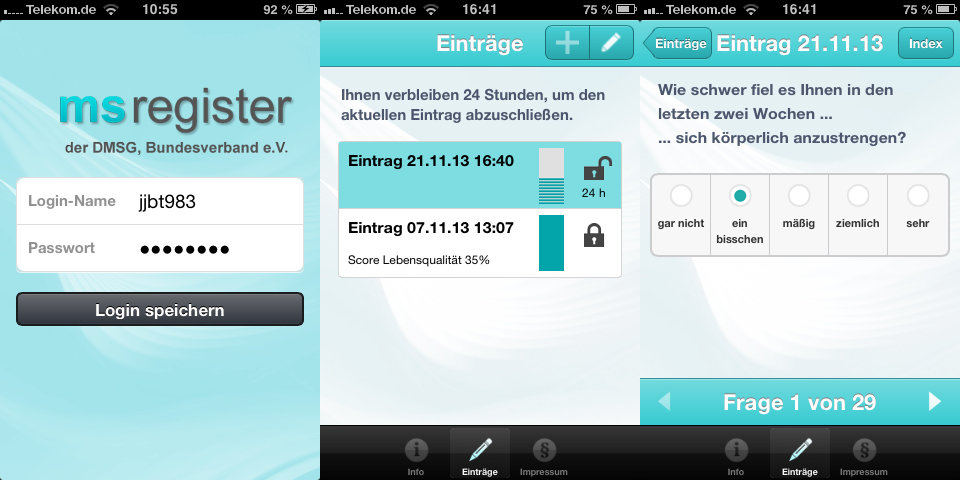
 A facility allowing study-relevant data to be easily and conveniently entered by the patients themselves had been requested for some time by our customers. In cooperation with the Department of Medical Informatics of the University Medical Center Göttingen under the direction of Prof. Dr. Otto Rienhoff, we developed a smartphone app for the MS register of the German Multiple Sclerosis Society (DMSG). The app can be used to enter data into secuTrial®. secuTrial® is our established system for clinical trial management and complies with the high national and international regulatory requirements and standards.
A facility allowing study-relevant data to be easily and conveniently entered by the patients themselves had been requested for some time by our customers. In cooperation with the Department of Medical Informatics of the University Medical Center Göttingen under the direction of Prof. Dr. Otto Rienhoff, we developed a smartphone app for the MS register of the German Multiple Sclerosis Society (DMSG). The app can be used to enter data into secuTrial®. secuTrial® is our established system for clinical trial management and complies with the high national and international regulatory requirements and standards.
The app offers users as well as clinical trial investigators a range of useful functions. For example, the entry times can be adjusted to the study plan by temporarily freezing the forms. A reminder system helps to ensure regular entries. Furthermore, to accommodate for various situations, the data can also be entered without Internet access. Upon connection, after successful authentication the encrypted data is transmitted to the system.
A pilot study of the DMSG focussing on patient-oriented documentation in the MS register is scheduled for 2014. The aim is to survey the quality of life of the study participants using the MSIS 29 questionnaire. During this period the app will undergo an evaluation phase. Afterwards, the plan is for it to be made widely available through the DMSG from next spring.