We recently developed four mobile secuTrial® patient apps for capturing patient-reported outcomes (PRO) in clinical studies. The design and user interface of each app were specifically tailored to the study and target group to ensure a smooth user experience and increase compliance. In terms of their use scenarios and required functions, the apps were very different. All of the apps are currently being used in studies.
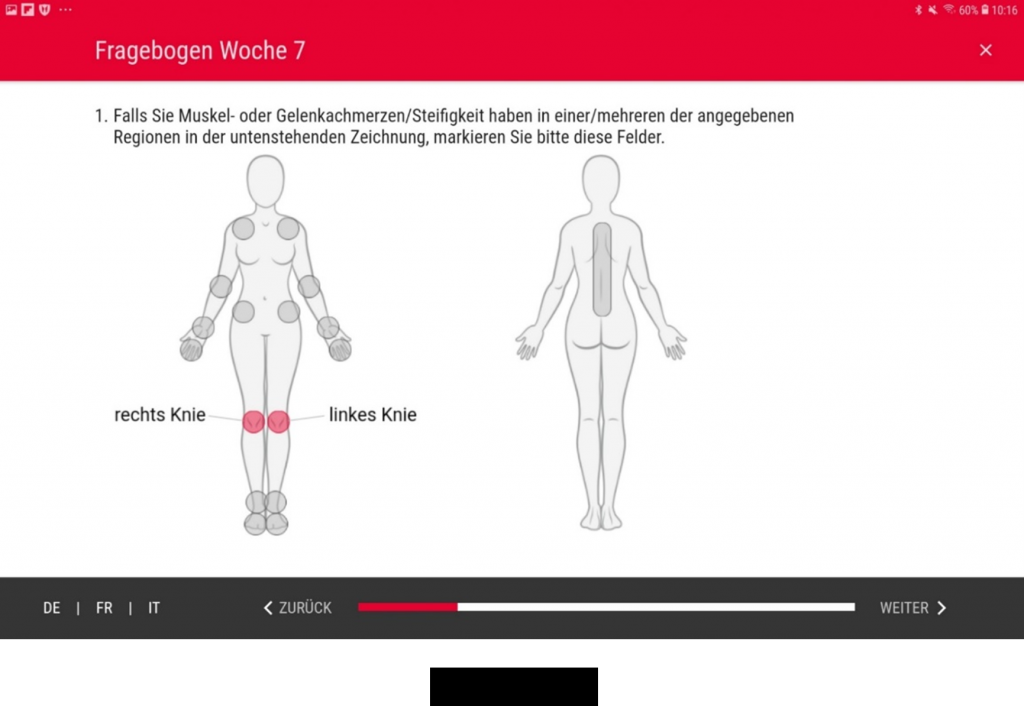
For the Swiss Group for Clinical Cancer Research (SAKK) we developed a secuTrial® tablet app which has been in use since July 2019. The study is being conducted on clinic-owned tablets which are handed to the patients for documentation in the clinic during a total of 11 visits. The app was developed and optimised specifically for this (Android) tablet model.
By February 2020, the app had been used by 107 patients with breast cancer. The study subjects are aged between 35 and 80 years old. Where necessary, the study staff help patients with the process of logging in to the app. Even older patients have no difficulties filling in the detailed quality of life questionnaires. According to Mr Schulenburg from SAKK, they are able to complete them easily “thanks to intuitive operation and the excellent design”. Only on very rare occasions have technical problems occurred in transferring data to secuTrial® as a result of an unstable internet connection.
Although they were invited to use the app, about half of the participating study centres still opted for conventional paper-based capturing of patient data. Further testing and practical experience will no doubt help to establish electronic capturing over the course of time.
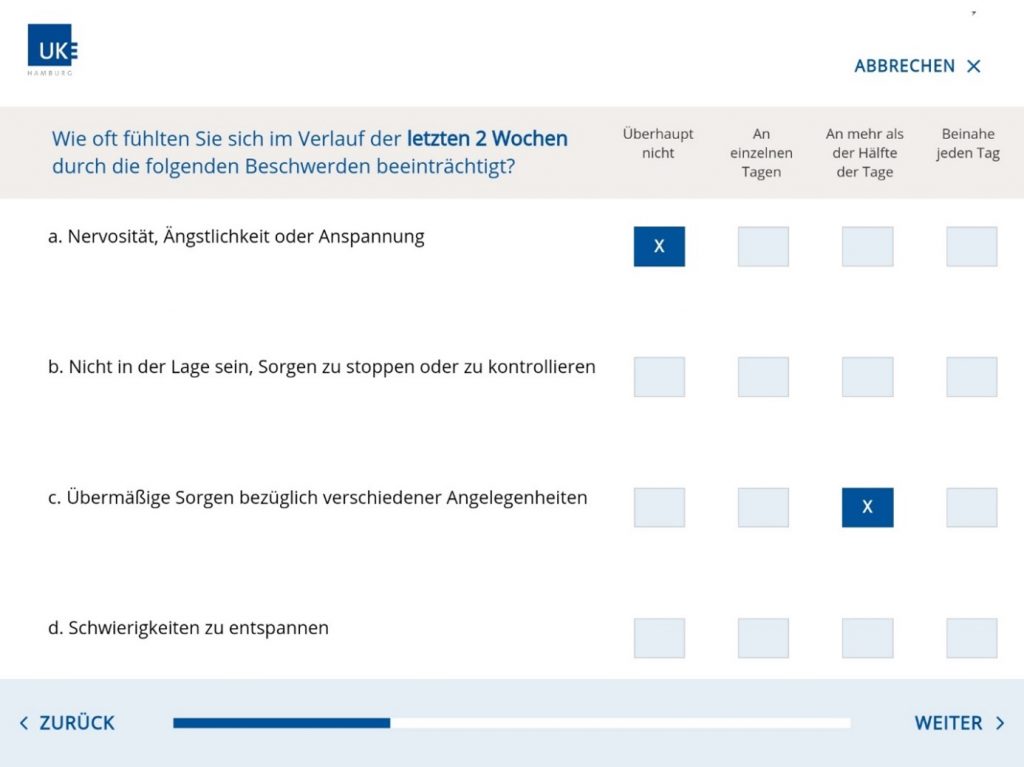
Another technically complex secuTrial® app was developed for CTC North (Hamburg) and its customer, the Clinic for Psychosomatic Medicine and Psychotherapy of the Medical Center Hamburg-Eppendorf. The app has been in use since November 2019 as part of a study on the early detection of depressive disorders, funded by the Innovation Committee of the Federal Joint Committee (G-BA). To date, the app has been used to recruit and screen over 6,000 subjects for depression in 50 GP surgeries. By July 2021, over 10,0000 people will be screened via the app.
The app was developed for offline use on (Android) tablets exclusively used for the study. Study centre staff in the GP surgery issue the tablets to patients after they have given their consent to participate in the study. The app users are a fairly heterogeneous group, ranging in age from 18 to 90. Thanks to the restrictive user guidance and large-scale layout, the app is easy to use even for older people. Technical questions and problems have only arisen on isolated occasions and were quickly resolved without requiring an app update.
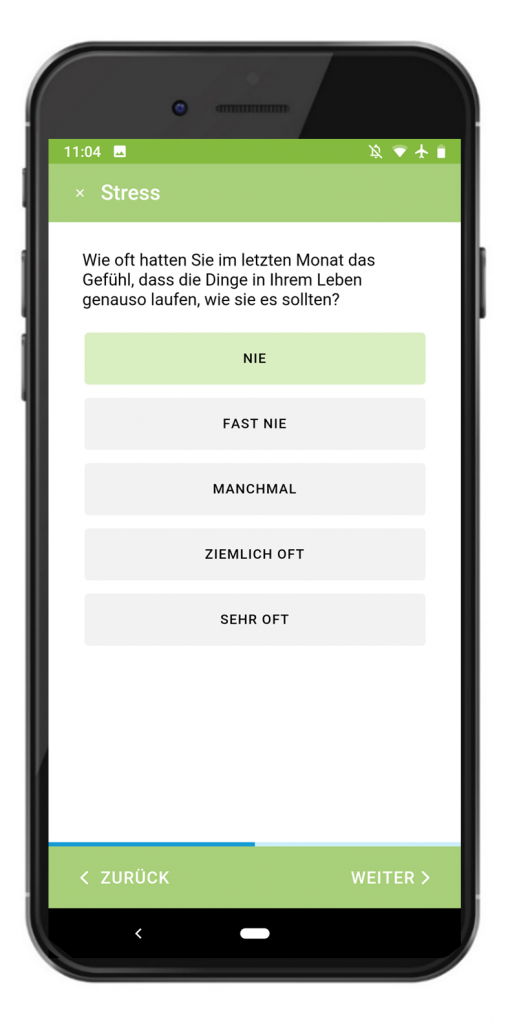
We also developed a secuTrial® smartphone app for the Gynaecology Clinic and Psychiatric Clinic of Universitätsklinikum Erlangen as part of a project funded by the Federal Ministry of Education and Research (BMBF). The app has been in use since March 2020 and is designed for capturing PRO in pregnant women. As the women use their own smartphones, we developed apps for iOS and Android which can be downloaded from the respective stores.
The women are recruited at the clinic. After giving their consent to participate in the study, they are given a brief introduction to the app and their login details. The patients then install the app independently at home and document a total of 31 visits over a period of 16 months. As an additional feature, the app offers mindfulness exercises that the women can do at any time and are part of the intervention. 107 women have been recruited so far (as at February 2021). The patients have been able to use the app without any issues and understand the structure and navigation. On the penultimate day of the 5-day data entry period for each visit, the users receive an entry reminder (local notification) on their smartphone. A small number of patients stated that they did not receive the reminders. A few patients also reported problems with logging in. However, after setting a new password, these issues were always able to be resolved.
As part of the TOTO study funded by the Federal Joint Committee, we developed a patient app for capturing patient-reported outcomes in cooperation with the University Medical Center Göttingen and Jena University Hospital. This was the first time that an app was based on the new secuTrial® Modern Data Capture, providing a highly comprehensive interface to secuTrial®. The app has been used reliably since November 2020 and has already been tested on multiple patients. Study participants are prompted by the app to document their sore throats weekly via push message.
We look forward to analysing the feedback from users.
We have also learned a lot during the development of the secuTrial® app and would like to share these insights with our customers even before we begin a new project together. We would therefore be pleased to advise you on the following points:
- Support: If patients are documenting independently at home, it is important to ensure adequate support (e.g. when patients forget their passwords, checking the settings for receiving notifications). As the app manufacturer, we also want to provide support because questions and errors inevitably arise when using apps which need to be addressed.
- Use of personal devices vs. use of uniform “study” devices:
Creating an app for just one specific “study” device is less expensive and involves fewer risks because the app only needs to be developed for one specific system (display size, operating system/version) and the study centre has control over all operating system updates (Android, iOS). If patients are using their own devices, the app must be compatible with as many (common) smartphones on the market as possible. This requires development for different platforms, observance of device/manufacturer-specific properties and extensive testing. Compatibility with future operating system versions (Android, iOS) is also essential.
- Updates: For longer study periods and use of patient-owned devices, app updates need to be planned because operating system updates (iOS, Android updates) and new devices inherently entail risks. When updating secuTrial®, compatibility with the individual apps must also be ensured.
Many of our clients see mobile apps as the future for capturing patient-reported outcomes. We’d be interested to know what you think. We look forward to hearing your views and will gladly answer any questions you may have.
Are you interested in finding out more or do you have any questions? Please feel free to get in touch with us.
Contact details interActive Systems